Immunotherapy/TKI Combinations Yield Efficacy Benefit in RCC
During a Morning Rounds discussion, experts in the field of renal cell carcinoma reviewed treatment updates and the effective use of immunotherapy plus TKIs.
The expert panel

Follow Wenxin Xu, MD, on his Morning Rounds with Patrick Curran, MSN, AGACNP, and Marina Kaymakcalan, PharmD, as they discuss a patient case of a 68-year-old woman with a diagnosis of clear cell renal cell carcinoma (RCC). The colleagues reviewed the currently approved treatment options in the space and also spoke about communication to achieve multidisciplinary care.
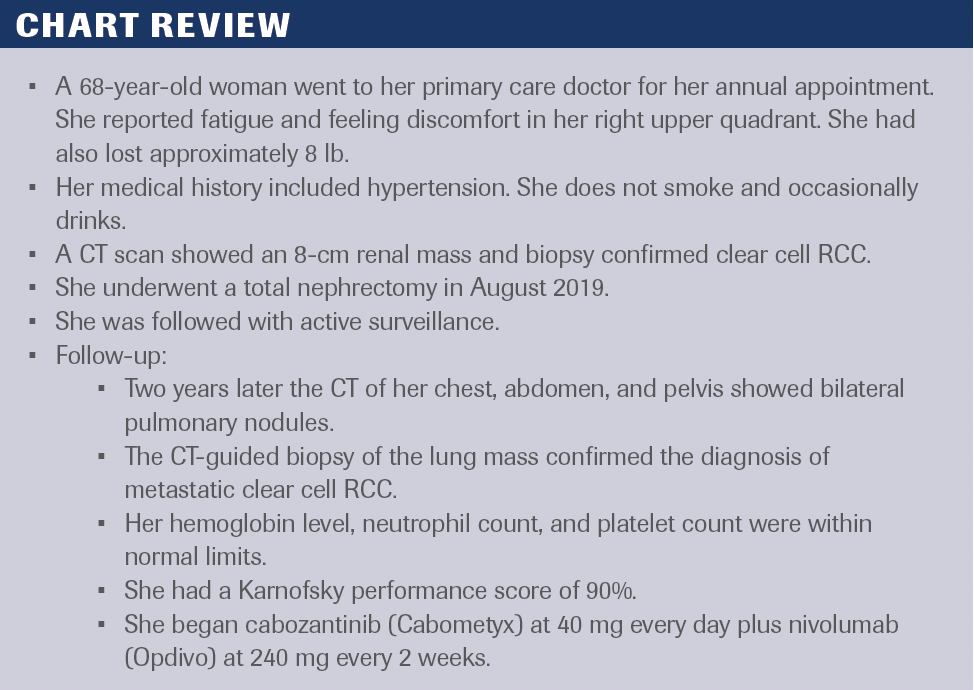
Chart Review
Xu: Let’s talk about some of the approved therapies for first-line treatment of kidney cancer. Currently, we have multiple randomized trials [whose data] have shown benefit for combination therapies in the first-line setting, including VEGF receptors, a tyrosine kinase inhibitor [TKI], and/or immunotherapy [IO] or combination immunotherapy, [which] have all shown benefits for progression-free survival [PFS] and overall survival [OS] compared with sunitinib [Sutent] alone.
Several of these combinations are now FDA approved in the first-line setting, including lenvatinib [Lenvima] and pembrolizumab [Keytruda], which showed both a PFS and OS benefit in the phase 3 CLEAR randomized trial [NCT02811861].1 In the phase 3 KEYNOTE-426 trial [NCT02853331], axitinib [Inlyta] and pembrolizumab showed a similar PFS and OS benefit.2 There is also cabozantinib and nivolumab [Opdivo], which also showed, again, both OS and PFS benefit in the phase 3 CheckMate 9ER trial [NCT03141177].3 There’s a single combination immunotherapy regimen that’s currently approved as ipilimumab [Yervoy] plus nivolumab, which was assessed in the phase 3 CheckMate 214 randomized trial [NCT02231749], but the FDA approval for combination ipilimumab plus nivolumab is limited to the intermediate- and poor-risk setting for first-line kidney cancer.4,5
There are a couple of other regimens that are worth mentioning. Axitinib and avelumab [Bavencio] are another IO/TKI combination that is FDA-
approved.6 It’s less commonly used because [data from] the randomized trial, the phase 3 JAVELIN Renal 101 [NCT02684006], did not show a clear OS benefit.7 Finally, not all patients can get IO safely. For patients who cannot get IO, single-agent TKIs such as cabozantinib are still a viable option.
Kaymakcalan: If patients are fit and eligible to receive combination therapy, then that should be the preferred option over a single agent. [Data from] all 3 of the first-line TKI/IO trials like CLEAR, KEYNOTE-426, and CheckMate 9ER…showed an OS benefit and longer PFS compared with sunitinib, the
[preferred] single-agent TKI.
Ipilimumab plus nivolumab also demonstrated superiority over sunitinib in the CheckMate 214 trial in that intermediate- and poor-risk patient population with even long-term durable response.
[The findings from] these studies also showed that if a more immediate response is needed in the setting of a more aggressive disease, then IO/TKI options would be best given so that it works a little more immediately. The selection for which combination of IO/TKI options, as we do have several, comes down to which TKI is best suited for your patient. For example, [tolerating treatment] is a big concern, and using the combination with axitinib may be the best [given] that it has a very short half-life and it can be stopped quickly. There are [fewer], generally speaking, dose-limiting toxicities compared with some of the other TKIs. Single-agent treatment may be an option for patients if they have contraindications or comorbidities that may preclude them from receiving a combination of 1 of the 2 treatment modalities. For example, if somebody has an uncontrolled
autoimmune condition and perhaps has had a solid organ transplant, they may not be the best candidate to receive IO.
Xu: The patient who is described, based on the clinical presentation, has International Metastatic RCC Database Consortium (IMDC) favorable-risk disease; she didn’t have a lot of laboratory abnormalities. It was over 2 years from the time of her diagnosis until she required the initiation of treatment. The IMDC risk score does help us to decide the kinds of measurements that may be appropriate for this patient.
When I think about a patient with IMDC favorable risk, the first question I ask myself is “Does the patient even need to start medication right away?” As you saw in this patient who initially had maybe very small lung nodules, asymptomatic and potentially slow-growing, active surveillance can be a reasonable option for some patients with IMDC favorable risk. Patients with active surveillance may be able to go 1, 2, or 3 years without needing to start medication. Alternatively, some patients have only 1 or 2 sites of disease. If there are 1 or 2 lung nodules or maybe some patients need treatment but do not have a lot of symptoms, maybe they have a disease burden that is relatively slow growing. Those patients may be candidates for even single-agent TKI therapy, especially if they have some reason that they want to avoid IO right away. If the therapy doesn’t work, those patients may be able to get IO in the second line, which we know still has an OS benefit.
Curran: The data highlight how important and how helpful the risk stratification of RCC can be in terms of informing treatment options. It’s important to also use your clinical judgment, and consider the patient’s values and their goals of care and a lot of their decision-making as well as helping them understand the data.
If you’re just going by the National Comprehensive Cancer Network guidelines for someone with favorable-risk disease, generally ipilimumab/nivolumab or combination IO wouldn’t be something you would typically consider.8 Maybe you have a patient who has some burden of disease or a lower burden of disease that you’re a little worried about, and they have sarcomatoid or rhabdoid features and may be having a prolonged treatment-free interval, or your patient wants some durable response to treatment. Then that aspect [of IO therapy] you might consider doing if you need to [find treatment] for somebody with a favorable-risk situation.
Kaymakcalan: It’s also important to remember that these are just general guidelines, and there is no one-size-fits-all approach to treating metastatic RCC. The best treatment for a particular patient will depend on their individual characteristics, including their risk profile, their symptoms, and their treatment goals. It is also important to consider the patient’s preferences and values when making treatment decisions.
Xu: One thing that I get asked almost every day by patients is what to do with supplements, including herbal supplements, as well as alternative medications that patients may be taking to try to help with either cancer-related symptoms or adverse effects. What do you tell patients when they ask about using these herbal supplements? What do we think about that?
Kaymakcalan: We are fortunate enough to be well versed in many herbs and supplements. There is growing literature around a lot of these other alternative treatments as well. One very important thing that we can do with our patients, and we’re fortunate enough to do [because] we have this multidisciplinary approach, is to have different clinicians asking the same type of questions. Sometimes we’re getting different answers. Perhaps when the patient first comes in, and the doctor who is meeting them for the first time is asking them about their medication, they may be thinking about other things. They may not mention some of the things that they’re [taking]. We can talk to them again, or somebody else can talk to them. Being a pharmacist in a clinic, I can also have a separate conversation with them, either that day or on a separate day. They can gather their thoughts and maybe have these things written down somewhere and can have a whole discussion with a lot of time to have a discussion around these alternative medications.
There are data out there to understand the possible drug interactions, toxicities, and expected adverse effects of some of these. We err on the side of caution. If there are data out there, we like to present that data to [the patients], especially if they’re compelled to be taking these alternative types of supplements and medicines. If we see them as not causing any potential issue with their cancer treatment, then we generally allow patients to continue these treatments that they might find useful, either with treating comorbidity or managing some symptoms that they may already have.
Now there are also data to support some of these issues that may arise with drug interactions, being that a lot of these things are oral [treatments]. A lot of these [drugs] are processed by the liver, so there’s a potential for some of these drug interactions. We can talk through that with the patient, and explain why we think that certain things need to be on hold while we are giving them other types of cancer treatment. We have the opportunity with the patient to engage in that discussion and to have them also understand what the totality of their care is about.
REFERENCES
1. Motzer RJ, Porta C, Eto M, et al. Final prespecified overall survival (OS) analysis of CLEAR: 4-year follow-up of lenvatinib plus pembrolizumab (L+P) vs sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2023;41(supp 16):4502. doi:10.1200/JCO.2023.41.16_suppl.4502
2. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib as first-line therapy for advanced clear cell renal cell carcinoma: 5-year analysis of KEYNOTE-426. J Clin Oncol. 2023;41(suppl 17):LBA4501. doi:10.1200/JCO.2023.41.17_suppl.LBA4501
3. Motzer RJ, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(7):888-898. doi:10.1016/S1470-2045(22)00290-X
4. Five-year data from CheckMate-214 show Opdivo (nivolumab) plus Yervoy (ipilimumab) demonstrates longest median overall survival currently reported in phase 3 trial of patients with previously untreated advanced or metastatic renal cell carcinoma. News release. Bristol Myers Squibb. September 16, 2021. Accessed November 6, 2023. https://bit.ly/3QlxWGy
5. FDA approves nivolumab plus ipilimumab combination for intermediate or poor-risk advanced renal cell carcinoma. FDA. April 16, 2018. Accessed November 6, 2023. https://bit.ly/45ZOutC
6. FDA approves avelumab plus axitinib for renal cell carcinoma. FDA. Updated May 15, 2019. Accessed November 6, 2023. https://bit.ly/471vk7J
7. Haanen JBAG, Larkin J, Choueiri TK, et al. Extended follow-up from JAVELIN Renal 101: subgroup analysis of avelumab plus axitinib versus sunitinib by the International Metastatic Renal Cell Carcinoma Database Consortium risk group in patients with advanced renal cell carcinoma. ESMO Open. 2023;8(3):101210. doi:10.1016/j.esmoop.2023.101210
8. NCCN. Clinical Practice Guidelines in Oncology. Kidney cancer, version 1.2024. Accessed November 6, 2023. https://bit.ly/470ffz8