Refractory Myeloma Responds to Proteasome Inhibitor
ORLANDO-The proteasome inhibitor MLN341 (formerly known as LDP-341 and PS-341) shows evidence of safety, biologic activity, and antitumor activity in the treatment of multiple myeloma, according to preliminary analysis of a phase II trial, Paul G. Richardson, MD, said at the 43rd Annual Meeting of the American Society of Hematology (abstract 3223). [The proteasome is an intracellular enzyme present in the cytoplasm and nucleus.]
ORLANDOThe proteasome inhibitor MLN341 (formerly known as LDP-341 and PS-341) shows evidence of safety, biologic activity, and antitumor activity in the treatment of multiple myeloma, according to preliminary analysis of a phase II trial, Paul G. Richardson, MD, said at the 43rd Annual Meeting of the American Society of Hematology (abstract 3223). [The proteasome is an intracellular enzyme present in the cytoplasm and nucleus.]
MLN341 (Millennium Pharmaceuticals, Cambridge, Massachusetts) is being tested in a variety of malignancies, but appears "especially promising" in multiple myeloma, Dr. Richardson said.
The agent has previously been shown to kill tumor cells directly and to induce complete tumor regression in animals, he said. Possible mechanisms of action in the treatment of myeloma include induction of apoptosis and disruption of the interaction of the microenvironment with myeloma cells, inhibiting binding of a myeloma cell to stromal cells.
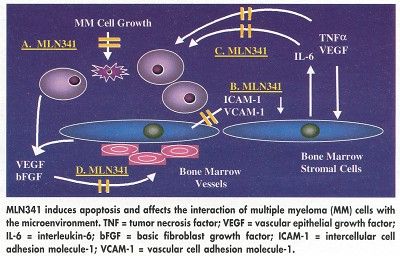
As a proteasome inhibitor, MLN341 affects the degradation of a large variety of intracellular proteins, but is selective and does not inhibit other enzymes. It reduces intercellular factor (IF)kappaB degradation by the proteasome, allowing more IFkappaB to bind to nuclear factor (NF)kappaB and so reducing NFkappaB levels.
NFkB, a transcription factor, drives myeloma cell growth in the microenvironment by upregulating the expression of key growth factors, including interleukin-6, vascular epithelial growth factor, and tumor necrosis factor-alfa.
The agent also inhibits degradation of proteins involved in cell cycle regulation, including p53 and p21 (CDK inhibitor), and may possibly inhibit angiogenesis, Dr. Richardson said.
Multicenter, phase II studies of MLN341 in multiple myeloma are ongoing, and the results so far are encouraging. Dr. Richardson, of the Dana-Farber Cancer Institute and Harvard Medical School, said that MLN341 was well tolerated, that studies have shown evidence that myeloma cells are 100 to 1,000 times more sensitive to MLN341 than are normal lymphocytes, and that patients refractory to other treatment modalities have shown good responses.
"This drug has a manageable toxicity profile, and we can administer it as outpatient therapy by IV push without premedication," Dr. Richardson said. "We ask investigators to watch patients for 1 hour after the infusion, but we have not seen a significant number of infusion-related side effects."
Study Protocol
The study reported by Dr. Richardson enrolled 200 patients with myeloma; the primary cohort enrolled 75 patients and the second cohort included 125 patients. Most patients had undergone multiple previous regimens (median, five).
Patients received MLN341 at 1.3 mg/m² by IV push over 3 to 6 seconds on days 1, 4, 8, and 11 of a 21-day cycle for up to eight cycles. The twice-weekly schedule was designed to allow for full recovery of the proteasome between doses. An extension protocol was made available for patients who responded, and patients continue on this therapy at the present time.
Patients with progressive disease after two cycles had the option of adding dexamethasone. MLN341 appears to augment the ability of regular chemotherapy, in general, and has specific additive effects with dexamethasone.
Among 93 patients evaluable for toxicity and safety, no grade 3-4 toxicities were observed in the majority of patients, Dr. Richardson reported. Side effects included diarrhea, hypotension, fatigue, and a mild decline in neutrophil and platelet counts. Peripheral neuropathy occurred in 17% of patients, but many had received prior neurotoxic drugs, Dr. Richardson said.
Majority Respond
In this preliminary analysis, the majority of patients treated responded to MLN341 (defined as a 25% or greater reduction in M protein after two cycles). Among 54 patients evaluable for efficacy analysis, 52% (28 patients) responded and another 33% (18) had stable disease. "Remarkably, there are complete or near complete responses even in this heavily pretreated population," Dr. Richardson said. The number of prior treatments was not predictive of response, and duration of therapy was important, with some patients responding after 70 to 80 days of treatment.
One patient with recurrent myeloma who had undergone 14 prior therapies, achieved a sustained complete remission with MLN341. Another patient, a 66-year-old man with refractory relapsed disease, was planning to enter hospice after a lack of response to VAD (vincristine, doxorubicin, dexamethasone), high-dose therapy and transplantation, and thalidomide (Thalomid). A month after starting MLN341, he felt "the best ever," Dr. Richardson said. "By the conclusion of treatment, he had painted his house and requested a prescription for Viagra."
Quality-of-life analysis from the phase II trial is ongoing, as well as genomic analyses "to define why some patients responded while others did not," Dr. Richardson said.
Investigators are planning a comparative, randomized, phase III trial of MLN341 in multiple myeloma in the United States and Europe, he said. A phase II trial is already underway in patients in first relapse comparing a dose of 1 mg/m² with the 1.3 mg/m² dose used in Dr. Richardson’s study. He said that the "best use" of MLN341 in the future would likely be in combination with conventional agents or possibly with new immunomodulatory drugs such as CC5013.