Predictive Biomarkers for Immunotherapy Response Beyond PD-1/PD-L1
ABSTRACT Advances in immuno-oncology over the last several years have led to FDA approvals of novel agents. As our understanding of immune response and its checkpoints has evolved, further advances have been made in treatment for several cancer types. To predict a response to immunotherapy, the initial biomarkers used were expression of the PD-1 receptor and PD-L1, as assessed by immunohistochemistry. More recently, predictive biomarkers have included microsatellite instability, DNA mismatch repair, and tumor mutational burden. Although these markers may be clinically relevant in predicting an immunotherapy response, cancer immunotherapy fails some patients. Improved understanding of the human immune system is necessary, as is a careful evaluation of the methods used to predict and assess response to Immuno-oncology treatments. With the application of therapeutic immune-modulating agents, more comprehensive assays, and associated bioinformatics tools to accurately assess the tumor microenvironment, we may better predict responses to immuno-oncology agents and the ever-increasing complexity of their clinical use.
Darabi is a clinical genomics scientist for the Precision Medicine Program at Hoag Family Cancer Institute.

Braxton is a molecular pathologist for the Precision Medicine Program at Hoag Family Cancer Institute.

Eisenberg is the executive medical director of the Hoag Family Cancer Institute and a professor of clinical surgery at the University of Southern California Keck School of Medicine.

Demeure is the program director for the Precision Medicine Program at Hoag Family Cancer Institute.

Background
Progress in cancer immunotherapy is advancing rapidly, primarily as the result of better understanding the complexities of immune system modulations, both in sickness and health. Discoveries in cancer immunotherapy and immuno-oncology’s successful application in various tumor types led to the award of the 2018 Nobel Prize in Physiology or Medicine to James P. Allison, PhD, and Tasuku Honjo, MD, PhD.1 The US FDA has approved several immuno-oncology agents since 2011.
Despite the notable successes, however, many patients don’t benefit from cancer immunotherapy, in part due to the integrated nature of the human immune system and its interaction with cancer cells.2
The hallmarks of cancer include genetic and epigenetic changes that lead to the transformation of normal cells, the interaction of these cancer cells with the
immune environment, and the cancer cells’ capability to evade the immune response.3 To clarify how immune resistance works, it is helpful to delineate the causative contributors to immune sensitivity and cancer. Antitumor activity of the immune system is considered to be cancer immunosurveillance, and this surveillance may be interrupted by evading the immune response or suppressing its effect.3,4 Careful assessment of the methods to predict and assess the response to immunotherapy beyond currently used assays is necessary. Improved understanding of the mechanisms involved in inducing and escaping tumor immune response—and especially the role of the tumor microenvironment—will inform the development of therapeutic agents.
The Cancer Immunity Cycle and Tumor Immune Microenvironment Phenotypes
The cancer immunity cycle is the process by which cancer cells release antigens that are processed and presented by antigen-presenting cells, with subsequent T-cell priming and activation. The activated T-cells then migrate out of the lymph nodes into systemic circulation; they traffic to the tumor site, infiltrate the tumor, and (perhaps) cause cytotoxic T-cell tumor killing.5 Each step in this process can be influenced by tumor intrinsic factors, host factors, host microbiome, environmental factors, and prior treatments.6 For instance, if a tumor does not produce antigens, then an immune response will fail to be initiated.7 Likewise, if a host cannot mount an immune response due to immunosenescence (age-related deterioration of the immune system), the tumor will evade eradication even if an abundant antigen is present.8
There are 3 tumor immune microenvironment phenotypes. First, inflamed (hot) tumors show a high degree of cytotoxic T-cell infiltration and elaborate proinflammatory cytokines. Higher response rates to anti–PD-1/PD-L1 therapy in inflamed tumors have been reported.6 While an inflamed microenvironment is thought to be necessary for effective checkpoint blockade, it is apparent that its presence alone does not guarantee clinical responses. Second, immune-excluded (warm) tumors show T-cells within the tumor stroma but little infiltration into the tumor. These peritumoral lymphocytes have been shown to be phenotypically distinct from tumor-infiltrating lymphocytes, and this microenvironment portends a diminished immunological and clinical response to anti–PD-L1/PD-1 therapy.6, 9-11 Third, immune desert (cold) tumors show few or no T-cells within the microenvironment; they are unlikely to respond to anti–PD-L1/PD-1 therapy.6 Understanding the components of the cancer immunity cycle and the tumor immune microenvironment phenotypes provide a framework for developing novel predictive biomarkers and treatment strategies within the immunotherapy development arena.
Among the most intriguing facets of the cancer immunity cycle currently being studied is the host systemic immune response. Every person has a homeostatic baseline of immune cell abundance and T-cell receptor diversity circulating within their peripheral blood, and this baseline differs significantly among individuals.12 This variation is overwhelmingly dictated by environmental factors, with only sparse contributions from heritable factors.12,13 Furthermore, as an individual ages, there are significant declines in naïve CD8+ T-cells and compensatory increases in central memory CD4+ T-cells.14 Because naïve CD8+ T-cells are the cell types necessary for recognizing new antigens, their decline is consistent with the observation of immunosenescence in older individuals.8 Immunosenescence also involves decreased T-cell receptor diversity15,16 and a broad decline in overall immune system activity. Seropositivity for cytomegalovirus (CMV) appears to significantly adversely impact the immune repertoire and may mediate immunosenescence; however, it is still unclear if CMV seropositivity impacts the effectiveness of immunotherapeutics.8,17
Significant evidence indicates that the diversity of the T-cell receptor repertoire is vital in the context of immune checkpoint blockade, or inhibition (ICI). The diversity and size of T-cell receptor clones responding to ICI have been shown to correlate with clinical responses and survival in patients with pancreatic cancer18 and metastatic melanoma.19 Similarly, peripheral lymphopenia has been shown to be present in 20% of patients with solid tumors, and it is associated with poor survival in multiple tumor types in the setting of ICI.20-22 Additionally, the amount of tumor present has been shown to be an important determinant of ICI failure,23 which suggests that in some patients, the immune system may be unable to expand sufficiently to accomplish tumor cell killing when faced with high tumor burden. This evidence supports the assertion that a significant proportion of patients inherently lack robust immune regulation and therefore will not benefit from current ICI strategies. Recent technological advances enabling measurement of the peripheral immune system and of the tumor immune microenvironment can inform strategies to replace an inadequate adaptive immune system with treatments such as cellular therapies (eg, chimeric antigen receptor T-cell therapy).
Even if a patient can mount a systemic immune response, the tumor may evade the immune system by a variety of tumor-intrinsic mechanisms. Alterations in oncogenic signaling pathways, including TP53, PTEN, MYC, beta catenin, and LKB1, have been shown to mediate immune evasion in a variety of cancers via manipulation of factors important in T-cell recruitment and infiltration.24 Similarly, tumors may elaborate cytokines unsuitable to support a cytotoxic T-cell–mediated immune response. In a post hoc analysis of the IMVIGOR trial (NCT02108652) of atezolizumab in metastatic urothelial carcinoma, high levels of systemic and tumor microenvironment interleukin-8, a cytokine that typically serves to attract neutrophils, were found to correlate with reduced benefit of the PD-L1 inhibitor.25 Failures of the immune system at the systemic and microenvironment levels likely lead to cold or warm tumor immune microenvironment phenotypes.
Known Biomarkers
Tumor immunotherapy increases the antitumor response by the patient’s immune system. This antitumor response involves different types of immune cells. In the past few years, ICIs have evolved and are now included in therapies for cancer types including non–small cell lung cancer (NSCLC), melanoma, bladder cancer, renal cell carcinoma, breast cancer, and endometrial cancer.26,27 ICIs target the key mediators of pathways that are involved in immune response. FDA-approved ICIs utilized for different tumor types include pembrolizumab, atezolizumab, avelumab, durvalumab, ipilimumab, and nivolumab. Several commercially available tests can identify biomarkers that indicate the level of response to immunotherapy. Detection of these predictive biomarkers assists in identifying patients for immunotherapy as well as those who may be resistant to such treatments.28
Cytotoxic T lymphocytes identify the tumor-associated antigens on cancer cells and destroy them. PD-1 is a membrane receptor protein on T-cells that induces cell death. PD-1 reduces antigen receptor activation by PD-L1 and PD-L2. PD-L1 presents on dendritic cells in activated macrophages. PD-1 plays an important role in the mechanism of immune escape by binding to PD-L1 or PD-L2 and activating the signaling pathway in cancer cells that express PD-L1.4
Various methods are used to detect the expression of PD-L1. Immunohistochemistry (IHC) assays may be useful in determining the response to immunotherapy. Antibody clones to identify and quantify PD-L1 protein are 22C3, SP142, 28-8, and SP263 that are registered at FDA on Dako and Ventata platforms.29 Different clinical trials that led to the approval of the immuno-oncology agents used different assays to assess PD-L1 expression, so it is unclear if these assays are similar. In addition, intratumor heterogeneity, technical issues, time of biopsy, and variation in interpretation of “positivity” are current challenges in PD-L1 assays.29
Besides PD-1/PD-L1 expression analysis, the presence of microsatellite instability (MSI) is an important response marker. It is characterized by deficient DNA mismatch repair (MMR). The DNA MMR pathway includes MLH1, MSH2, PMS2, and MSH6 proteins, and when the pathway is defective (dMMR), it can be a measure to select patients for immunotherapy (Table).
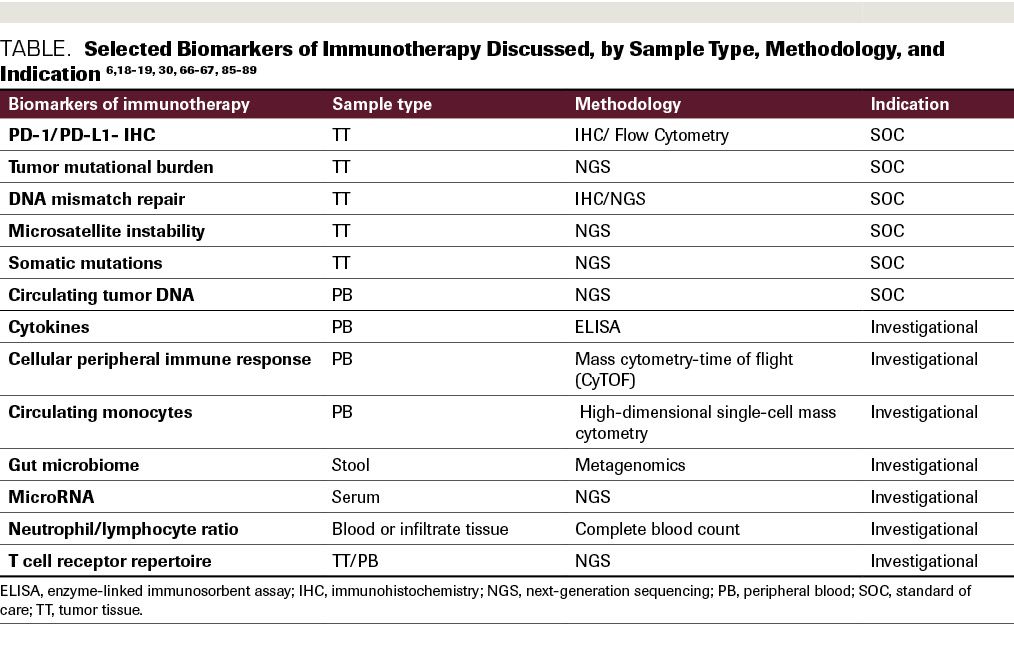
Table. Selected Biomarkers of Immunotherapy Discussed, by Sample Type, Methodology, and indication
Tumor Mutational Burden
A biomarker frequently used to predict whether a tumor might respond favorably to treatment with anti–PD-1 or anti–PD-L1 immunotherapy is tumor mutational burden (TMB), defined as “a quantitative measure of the total number of somatic nonsynonymous mutations within the coding region of a tumor genome.”30 The hypothesis is that tumors exhibiting high TMB will expose more neoantigens that are capable of eliciting an immune response. Initially, TMB was determined using whole exome sequencing (WES) derived by next-generation sequence analysis of tumor samples and matching nontumor tissue. It is important to understand the evidence to support the use of TMB, the techniques by which TMB is determined, and the limitations associated with this biomarker.
WES is uncommon in real-world practice. Rather, TMB is determined by interpreting data from large tumor sequencing panels that include only somatic genes. Determining TMB from such a panel is a normalized extrapolation expressed as the number of mutations per megabase (mut/Mb); however, there is variability among labs in how this is performed and interpreted.31 Differences arise in sample preparation and in sequencing techniques such as panel design, coverage and depth, bioinformatics pipelines, thresholds for variant calling, and cutoffs for calling high TMB; these have all differed in studies reporting TMB as a biomarker of response to ICI. Matched tumor–normal sequencing allowed for accurate somatic variant calls among these studies, although fewer labs sequence normal matching DNA. Most, but not all, commercial labs used in clinical practice sequence large panels, each consisting of 300 to 600 genes. Panels often cover the entire coding regions, or a relevant regions of genes, or hotspots of known mutations. Large-panel sequencing does have advantages, including lower cost, decreased DNA requirements, and more rapid results when compared with WES. Therefore, when one must sequence a patient’s paraffin-embedded (often small) tumor samples to provide timely results to guide therapeutic decisions, gene panels may be the best option.
Recent analysis suggests that targeted somatic gene panels, if they are sufficiently large (sequencing at least 315 genes, or 1.1 Mb), do correlate well with TMB as determined by WES.32 However, the correlation may not hold equally well across all tumor types. Examination of FoundationOne CDx data compared with The Cancer Genome Atlas (TCGA) data for 33 tumor types determined that TMB estimation was reliable (R2 ≥0.75) in just 24 of 33 TCGA cancer types.33 After removing the cases of ultra-high TMB or hypermutated tumors, the correlation was worse. The reliability of TMB calls based on FoundationOne CDx was deemed adequate in melanoma, lung adenocarcinoma, cervical squamous cell cancer, head and neck squamous cell cancer, and stomach adenocarcinoma. In several other tumor types, though, the panel test was found to inadequately estimate TMB. Overall, across all tumor types studied, a meta-analysis of published critical trials examining the use of immuno-oncology agents showed a 47% risk reduction for death in patients whose tumors had high TMB when compared with those with low TMB.34 It is not clear, however, that all individual cancer types adhere to this observation. Cutaneous melanoma and NSCLC are most studied, and this may be because these tumors, compared with most others, have higher overall TMB.35
Melanoma
Malignant melanoma is among the tumor types exhibiting the highest TMB, likely due to the role of DNA damage caused by ultraviolet light in the pathogenesis of these tumors.36 Several studies have shown that patients whose melanoma tumors exhibit high TMB have prolonged survival when treated with immunotherapy drugs.37 Snyder et al (2014) analyzed the exomes of 64 patients with malignant melanoma and demonstrated that patients treated with ipilimumab whose tumors harbored more than 100 nonsynonymous mutations per exome had improved survival compared with those with fewer mutations (P = .04).38 They used a bioinformatics pipeline that incorporated patients’ human leukocyte antigen (HLA) type, major histocompatibility complex (MHC) class I binding prediction, and modeling of T-cell receptor binding to develop a neoepitope signature that was highly correlated with survival (P = .002). Retrospective analysis of archival samples from an initial and a validation cohort representing a total of 65 patients using the FoundationOne CDx 315-gene panel showed that TMB was significantly higher in responders to anti–PD-1/PD-L1 therapy than in nonresponders (P <.005).39 In the validation cohort, the responders’ tumors had a median of 37.1 mut/Mb compared with 12.8 in nonresponders (P = .002). Both progression-free survival (PFS) and overall survival (OS) were better for the group of treated patients whose tumors had a high TMB. A recent review by Krieger et al cited 40 studies that present outcome data for melanoma.40 Of these, only 3 studies reported TMB as a biomarker, including the study by Johnson cited previously. One of the retrospective studies (Yaghmour et al, 2016) included a small number of patients and used different commercial sequencing panels, but no difference in OS for patients with melanoma was observed, perhaps due to relatively small numbers (P = .17).41 The other study (Roszik et al, 2016) predicted TMB from the 312 patients from the TCGA melanoma dataset.42 Results of another study(Forschner et al, 2019) showed that TMB in circulating tumor DNA (ctDNA) was higher in the patients who responded to combined PD-1 and CTLA-4 blockade.43 These investigators used a 710-gene panel in which all exonic and flanking intronic regions were sequenced. A high TMB was defined as more than 23.1 mut/Mb. Interestingly, the authors found that a decrease in ctDNA to less than 50% was favorably associated with improved survival. A high predicted TMB correlated with survival in patients treated with ipilimumab or adoptive T-cell therapy. In melanoma, a low TMB does not preclude response to PD-1/PD-L1 response, and in and of itself, TMB has a poor predictive value in distinguishing patients whose tumors will or will not respond to ICI therapy. 44
Lung Cancer
In 2015, the first study to show benefit from PD-1 blockade in NSCLC was KEYNOTE-001 (NCT01295827), in which 495 patients were treated with pembrolizumab. The benefit was correlated with PD-L1 expression of greater than 50% in the tumors; however, TMB was not assessed or reported.45 Also in 2015, Rivzi et al reported that patients whose tumors had higher somatic nonsynonymous mutational burden by WES had more durable clinical responses to treatment with pembrolizumab.46 Durable clinical benefit (DCB) was defined as a complete or partial response or stable disease for 6 or more months. In their study, 73% of patients whose tumors had a high TMB (above the median) had DCB, whereas only 13% of those with low TMB exhibited a benefit (P = .04).
For patients with advanced NSCLC, high TMB assessed with targeted large gene panels also seems to predict durable clinical benefit with PD-1 or PD-L1 blockade.47 In this series, the benefit was associated with a TMB above the 50th percentile of the study cohort and was independent of PD-L1 IHC findings. Moreover, other findings, including variants in EGFR or STK11, mitigated the benefit of immunotherapy. Further, patients with high TMB and strong IHC staining for PD-L1 fared the best.
TMB also added predicted response beyond PD-L1 status in the CheckMate 026 (NCT02041533) clinical trial, in which 541 patients with previously untreated stage IV NSCLC and a PD-L1 tumor expression of at least 1% were randomly assigned to receive either nivolumab or standard-of-care chemotherapy. OS was similar in both cohorts, although the toxicity profile was better for the group of patients receiving nivolumab.48 Post hoc analysis showed that the subset of patients with both PD-L1 expression more than 50% and high TMB had a greater likelihood of response to nivolumab, faring better than patients whose tumors exhibited only 1 or neither of these characteristics. Also of note, Chang et al retrospectively assessed TMB in 44 of the patients in this study using the FoundationOne CDx assay and compared this with the findings obtained from WES.49 A very strong correlation (Spearman’s r = 0.90) existed between the results obtained from both methods. The CheckMate 227 study (NCT02477826) demonstrated that patients fared better with dual ICI using nivolumab and ipilimumab than they did with chemotherapy.50 Improved overall tumor response and survival in NSCLC was seen in patients regardless of whether their tumors had more or fewer than 10 mut/Mb sequenced using the FoundationOne CDx assay.
Not all studies support TMB as an independent biomarker predictive of a favorable response to ICI. Most do, however, support the predictive value of TMB, and recently published reviews and meta-analyses conclude that TMB is a valid biomarker.37,51,52 In April 2020, the FDA granted Fast Track designations for immunotherapeutic agents for metastatic or recurrent NSCLC as well as for urothelial, cervical, and central nervous system cancers based on a high TMB. In addition, in June 2020, the FDA approved pembrolizumab for children and adults with high TMB (≥10 mut/Mb) solid tumors.
Other Tumors
The recent FDA approvals for immunotherapy in advanced renal cell carcinoma, and interest in this application, have led to clinical trials that compare different treatment options and the optimal biomarkers for immunotherapy. Somatic mutation data analysis from TCGA in 366 clear cell renal cell carcinoma tumors showed that higher TMB was associated with poor survival in this cohort. The most frequent somatic mutations were in VHL, BAP1, PBRM1, and SETD2 genes.53 In the CheckMate 214 study (NCT02231749), patients with renal cell carcinoma were randomized to take the combination of nivolumab plus ipilimumab or monotherapy with sunitinib; 41% in the combination arm achieved an objective response compared with 34% in the sunitinib arm. 54 In another study, KEYNOTE-426 (NCT02853331), the combination of axitinib and pembrolizumab was compared with sunitinib; the objective response was 59.3% with the combination vs 35.7% with sunitinib.55
The relationship between TMB and response to immunotherapy in bladder cancer was analyzed based on tumor immune microenvironment and tumor genome. Patients with high TMB in their tumors had significantly improved survival compared with the low-TMB group.56 Another research group assessed the association between TMB and prognosis in bladder cancer through TCGA data analysis, which showed that patients with high TMB have a better prognosis with immunotherapy than those with low TMB.57 As reported by Thomas et al in 2018, patients with breast cancer whose tumors show high TMB have longer survival.58 In a study of patients with colorectal cancer (CRC), however, low TMB correlated with favorable PFS but the association did not reach statistical significance.59 Finally, in a large pooled analysis of more than 10,000 patients with various tumor types receiving immuno-oncology treatments, authors assessed the relationship between TMB and clinical outcome, concluding that patients with high TMB had better OS when they received immunotherapy versus chemotherapy regardless of tumor type.60
Mismatch Repair Deficiency, Hypermutation, and Immuno-Oncology
Hypermutated tumors appear to be responsive to treatment with immuno-oncology agents. Hypermutation occurs when proofreading mechanisms of DNA replication are impaired, resulting in an accumulation of large numbers of replication errors. DNA mismatch repair would normally correct most of these errors; however, some patients have defects in MMR genes. Lynch syndrome, for instance, is characterized by an autosomal inherited heterozygous mutation in one of the MMR genes: MLH1, MSH2, MSH6, and PMS2. 61 Lynch syndrome may also be due to the deletion of the 3’ exons of the TACSTD1 gene encoding EPCAM. 62 MSI-H could be caused by hypermethylation of the MLH1 promoter.63 Hypermutation may also be observed in the setting of germline or somatic mutations in the DNA POLE or POLD1 genes.64,65 Patients with Lynch syndrome are at an increased risk for CRC, particularly at young age, as well as for cancers of the endometrium, ovary, stomach, small bowel, pancreas, biliary tract, ureter, renal pelvis, brain, and sebaceous glands.66
Because dMMR CRCs have high TMB, a trial using pembrolizumab was conducted in 41 patients, including 32 with metastatic CRC. Patients were treated with pembrolizumab (10 mg/kg of body weight) every 14 days. The overall response rate (ORR) was 40% in patients with dMMR tumors and 0% for those whose tumors were MMR proficient. PFS was associated with high TMB (P = .02). WES showed a mean of 1782 somatic mutations in MSI-H/dMMR tumors compared with only 73 observed in MMR-proficient tumors.67 A subsequent study has validated the observation across multiple tumor types, with a 53% response rate and 21% complete response rate seen in patients with advanced dMMR tumors.68
In May 2017, the FDA approved pembrolizumab for the treatment of tissue-agnostic metastatic or unresectable MSI-H/dMMR cancers that had progressed on prior treatment or for which no satisfactory treatment options exist. The approval was based on several studies involving patients with 15 different cancer types, including 149 patients with CRC, and it was the first cancer-site–agnostic FDA approval. One of the studies leading to this approval was the important KEYNOTE-016 trial (NCT01876511),67 and another was KEYNOTE-158 (NCT02628067), which enrolled 233 patients with 27 different non-CRC cancer types that were all MSI-H/dMMR.69 The ORR to treatment with pembrolizumab was 34%, and one-third of the responding patients had a complete response. Furthermore, 77.6% of these had response durations of more than 24 months.48 In an analysis of cBioPortal data, patients with mutations in POLE or POLD1 across all tumor types who were treated with ICIs had improved OS compared with patients who did not harbor one of these mutations. In the study’s multivariable Cox regression analysis controlling for MSI status and tumor type, the presence of a POLE or POLD1 mutation was an independent predictor of a benefit for ICI treatment (P = .047; HR, 1.41; 95% CI, 1.00-1.98).70
Emerging Biomarkers and Future Directions
As our knowledge evolves and grows, we will be better able to understand the biomarkers that inform the response to immunotherapy agents. We already understand that certain mutations may inhibit ICI. In nonsquamous NSCLCs that are EGFR and ALK wild type, mutations in STK11/LKB1 predict primary resistance to pembrolizumab, in that the addition of this agent did not improve outcomes associated with platinum doublet chemotherapy.71 This finding supports the lack of immuno-oncology drug benefit in KRAS-mutated lung adenocarcinomas with STK11/LKB1 comutations. The STK11/LKB1 gene encodes a serine-threonine kinase that, when inactivated through mutation, is associated with an immunologically inert (cold) tumor immune microenvironment with decreased infiltration of CD8+ T lymphocytes.72,73 Lung cancers that exhibited both KRAS and STK11/LBK1 mutations were most resistant to PD-1 ICI, with only 7.4% of patients deriving a response.74 In this study, patients with wild-type STK11/LBK1 tumors that harbored KRAS mutations had an ORR of 28.6%. The investigators also noted that tumors with a STK11/LBK1 mutation were more likely to be PD-L1–negative, although patients with PD-L1–positive, STK11/LBK1-mutated tumors still failed to respond to PD-1/PD-L1 blockade.
Other genomic events also appear to result in acquired resistance to immune therapy. For example, biallelic loss of PTEN was first reported in a patient with metastatic uterine leiomyosarcoma who had a complete remission on pembrolizumab but then developed an acquired resistance.75 This has been proposed as a mechanism of resistance in melanoma as well.76 Also in melanoma, loss of B2M77 and loss-of-function mutations of the JAK1 or JAK2 genes78 are thought to be mechanisms of acquired resistance. In a study by
Zaretzki et al, 4 patients with melanoma had initial responses to immunotherapy with pembrolizumab but then developed late resistance.Samples of their tumors from before and after treatment were evaluated by whole exome and transcriptome sequence analysis. It was found that one patient developed a nonsense inactivating mutation in the JAK1 gene that was not seen in the original tumor sample. Another patient’s tumor had acquired a splice site variant in the gene encoding JAK2. RNA sequencing confirmed that both mutations resulted in a truncated protein lacking the kinase domain. This makes sense, considering that JAK 1 and JAK2 gene products are thought to be necessary for interferon signaling and response.79 A third patient developed a frameshift deletion in B2M, resulting in impaired MHC class I cell membrane localization. No acquired mutation could be identified in the fourth patient studied. The study suggests that JAK1 and JAK2 loss-of-function mutations may confer resistance to ICI in other tumor types as well.80
Studies such as these, with clear biologic correlates, will continue to be important to improve the understanding of how genomic events modulate tumor immunogenicity. We know that not all mutations are equally immunogenic; only a minority of somatic mutations result in neoantigens that are presented in the context of MHC to activate T-cells.81,82 Knowing a patient’s HLA type and peptide binding motifs allows for predictions of mutated tumor-derived peptides, which can also inform the potential immunogenicity of tumors. This may be even more relevant when combining cellular therapy and immuno-oncology with several agents currently in the pipeline.83 Different types of available cellular-based treatments include CRISPR-engineered T-cells or ɣδT-cells, chimeric antigen receptor (CAR) T-cells, and macrophage-based therapies; most of these treatments are autologous.
In addition to the markers that can be identified through tumor testing, additional biomarkers from peripheral blood can and, some research studies suggest, should be assessed, although the value of some is still unclear. For instance, interferon-ɣ is overexpressed and is associated with clinical response in patients with melanoma, but the same result was not reported in NSCLC or renal cell carcinoma.84 Bioinformatics tools and high-dimensional single-cell mass cytometry were used to analyze and characterize a subset of immune cells in patients with advanced-stage melanoma before and after immunotherapy. The investigators reported that the frequency of circulating monocytes, CD14+CD16-HLA-DRhi, was a strong predictive factor in the success or failure of immunotherapy.85 High-resolution assays of the peripheral immune response and tumor microenvironment may one day enable us to fully apply the precision medicine paradigm to immuno-oncology. These assays include single-cell RNA sequencing, mass cytometry time-of-flight (CyTOF), high-resolution T-cell receptor repertoire sequencing, and tumor DNA WES. For instance, Subrahmanyam et al used CyTOF to find predictive markers for anti–PD-1 and anti–CTLA-4 profiling in peripheral blood from patients with melanoma. The authors concluded that CD8+ and CD4+ memory T-cells are important in response to anti–CTLA-4 therapy and could be potential biomarkers.86
A gut microbiome profile could be another indication of response to treatment. For example, Akkermansia muciniphila presence in stool samples is associated with a better outcome in NSCLC and renal cell carcinoma.87 It is unclear whether manipulation of the microbiome might improve response to immunotherapy. Furthermore, the ratio of neutrophil to lymphocyte and absolute lymphocyte count could be measured before treatment in patients with metastatic disease to assess response to ipilimumab. A high neutrophil/lymphocyte ratio (≥4) is an indication of poor prognosis.88 Epigenetic markers could also potentially be a prognostic marker in immunotherapy. Investigation of circulating microRNA as a predictive marker in NSCLC showed a 7-miR signature that is associated with outcome in patients who were treated with nivolumab.89 Many of these assays, and the advanced bioinformatic algorithms that produce the assay outputs, have not yet been put through the rigorous validation procedures necessary for deployment into the clinical laboratory (Table).
Further, novel imaging studies to monitor effector T-cell trafficking may find a place in the rapid assessment of treatment efficacy. One such modality developed by ImaginAb (Inglewood, CA) uses immunoPET scans with Zr-89 radiolabeled antibodies to CD8+ T-cells to assess immune-cell trafficking against tumor cells. To date, a phase 1 dose-escalation study demonstrated safety and established the effective dose and the optimal PET imaging protocols. Additional studies, including a phase 2 trial, are ongoing to demonstrate the utility of CD8 immunoPET as a predictive marker for immunotherapy. Once validated, implementing these assays and imaging modalities into routine clinical practice will bring new practical challenges in interpreting the results of, and acting on, these immune assays.
Discussion
Numerous challenges remain in immuno-oncology, including the determination of the drivers of cancer immunity, preclinical model design, early-phase trials, immune escape, and many more as elucidated by Hedge et al (2020).2 Currently, however, the methods to determine if immunotherapy would be effective are limited to PD-1/PD-L1 expression, MSI, MMR, and TMB status through IHC and next-generation sequencing, with the preference of paraffin-embedded tumor samples. When tumor sample is not readily available, or perhaps for sequential monitoring, assessment of TMB from ctDNA in plasma samples is possible and appears to correlate with findings seen from the analysis of tumor samples by WES.90 However, the optimal determination of TMB remains the WES of paired tumor and normal germline DNA in order to call true somatic variants. As technology evolves and expressed variants can be determined, transcriptome analysis may supplant exome sequencing. Furthermore, bioinformatics techniques will continue to improve, allowing for improved analytics.
Other novel immunotherapy agents are in the pipeline, under investigation to target different biomarkers. For example, a coinhibitory molecule called TIGIT—T-cell immunoreceptor with immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain—is involved in immunosurveillance and is upregulated in certain cancers. Anti-TIGIT antibodies are in the pipeline as immunotherapy agents, to be used as monotherapy or in combination with anti–PD-1/PD-L1 antibodies; they have shown synergy when combined. 91 Poliovirus receptor-related immunoglobulin domain-containing protein (PVRIG) is another immuno-inhibitory receptor where antagonist antibodies to inhibit PVRIG is currently in development. COM701 is an anti-PVRIG antibody that is currently under investigation.92
Evidence is clearly driving the field toward increasingly utilizing high-complexity laboratory testing to help select and monitor patients being treated with immunotherapeutics. A deep understanding of different mechanisms in immune response, and of potential biomarkers to assess sensitivity to immunotherapy, is crucial in the continuing effort to drive clinical benefit in patients with cancer.
Financial Disclosure: The authors have no significant financial interest in or other relationship with the manufacturer of any product or provider of any service mentioned in this article.
References
- The Nobel Prize in Physiology or Medicine 2018. News release. The Nobel Prize; October 10, 2018. Accessed March 25, 2020. https://www.nobelprize.org/prizes/medicine/2018/press-release/
- Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52(1):17-35. doi:10.1016/j.immuni.2019.12.011
- Allard B, Aspeslagh S, Garaud S, et al. Immuno-oncology-101: overview of major concepts and translational perspectives. Semin Cancer Biol. 2018;52(Pt 2):1-11. doi:10.1016/j.semcancer.2018.02.005
- Chamoto K, Hatae R, Honjo T. Current issues and perspectives in PD-1 blockade cancer immunotherapy. Int J Clin Oncol. 2020;25(5):790-800. doi:10.1007/s10147-019-01588-7
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1-10. doi:10.1016/j.immuni.2013.07.012
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321-330. doi:10.1038/nature21349
- Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168(4):707-723. doi:10.1016/j.cell.2017.01.017
- Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19(1):10-19. doi:10.1038/s41590-017-0006-x. Published correction appears in Nat Immunol. 2018;19(10):1146.
- Simoni Y, Becht E, Fehlings M, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557(7706):575-579. doi:10.1038/s41586-018-0130-2
- Jerby-Arnon L, Shah P, Cuoco MS, et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell. 2018;175(4):984-997.e24. doi:10.1016/j.cell.2018.09.006
- Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. 2015;348(6230):74-80. doi:10.1126/science.aaa6204
- Britanova OV, Putintseva EV, Shugay M, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192(6):2689-2698. doi:10.4049/jimmunol.1302064
- Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160(1-2):37-47. doi:10.1016/j.cell.2014.12.020
- Zalocusky KA, Kan MJ, Hu Z, et al. The 10,000 immunomes project: building a resource for human immunology. Cell Rep. 2018;25(2):513-522.e3. doi:10.1016/j.celrep.2018.09.021
- Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174(11):7446-7452. doi:10.4049/jimmunol.174.11.7446
- Britanova OV, Putintseva EV, Shugay M, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192(6):2689-2698. doi:10.4049/jimmunol.1302064
- Kaczorowski KJ, Shekhar K, Nkulikiyimfura D, et al. Continuous immunotypes describe human immune variation and predict diverse responses. Proc Natl Acad Sci U S A. 2017;114(30):E6097-E6106. doi:10.1073/pnas.1705065114
- Hopkins AC, Yarchoan M, Durham JN, et al. T cell receptor repertoire features associated with survival in immunotherapy-treated pancreatic ductal adenocarcinoma. JCI Insight. 2018;3(13):e122092. doi:10.1172/jci.insight.122092
- Fairfax BP, Taylor CA, Watson RA, et al. Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med. 2020;26(2):193-199. doi:10.1038/s41591-019-0734-6
- Ménétrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer. 2019;7(1):85. doi:10.1186/s40425-019-0549-5
- Galli G, Poggi M, Fucà G, et al. Basal lymphopenia as a potential predictor of benefit from immunotherapy in metastatic non-small cell lung cancer. Ann Oncol. 2018;29(suppl 8):viii493-viii547. doi:10.1093/annonc/mdy292
- Chen D, Verma V, Patel RR, et al. Absolute lymphocyte count predicts abscopal responses and outcomes in patients receiving combined immunotherapy and radiation therapy: analysis of 3 phase 1/2 trials. Int J Radiat Oncol Biol Phys. Published online February 7, 2020. 2020;S0360-3016(20)30158-9. doi:10.1016/j.ijrobp.2020.01.032
- Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60-65. doi:10.1038/nature22079
- Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer. 2018;18(3):139-147. doi:10.1038/nrc.2017.117
- Yuen KC, Liu LF, Gupta V, et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat Med. 2020;26(5):693-698. doi:10.1038/s41591-020-0860-1
- Liu X-H. Research progress in clinical efficacy evaluation of tumor immunotherapy. Precis Med Res. 2020;2(1):33-37. doi:10.12032/PMR201900025
- Martin JD, Cabral H, Stylianopoulos T, Jain RK. Improving cancer immunotherapy using nanomedicines: progress, opportunities and challenges. Nat Rev Clin Oncol. 2020;17(4):251-266. doi:10.1038/s41571-019-0308-z
- Touat M, Li YY, Boynton AN, et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature. 2020;580(7804):517-523. doi:10.1038/s41586-020-2209-9
- Hunter KA, Socinski MA, Villaruz LC. PD-L1 testing in guiding patient selection for PD-1/PD-L1 inhibitor therapy in lung cancer. Mol Diagn Therapy. 2018;22(1):1-10. doi:10.1007/s40291-017-0308-6
- Meléndez B, Van Campenhout C, Rorive S, et al. Methods of measurement for tumor mutational burden in tumor tissue. Transl Lung Cancer Res. 2018;7(6):661-667. doi:10.21037/tlcr.2018.08.02
- Stenzinger A, Allen JD, Maas J, et al. Tumor mutational burden standardization initiatives: recommendations for consistent tumor mutational burden assessment in clinical samples to guide immunotherapy treatment decisions. Genes Chromosomes Cancer. 2019;58(8):578-588. doi:10.1002/gcc.22733
- Chalmers ZR, Connelly CF, Fabrizio D, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017;9(1):34. doi:10.1186/s13073-017-0424-2
- Wu HX, Wang ZX, Zhao Q, et al. Designing gene panels for tumor mutational burden estimation: the need to shift from ‘correlation’ to ‘accuracy.’ J immunother Cancer. 2019;7(1):206. doi:10.1186/s40425-019-0681-2
- Kim JY, Kronbichler A, Eisenhut M, et al. Tumor mutational burden and efficacy of immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers (Basel). 2019;11(11):1798. doi:10.3390/cancers11111798
- Alexandrov LB, Kim J, Haradhvala NJ, et al; PCAWG Consortium. The repertoire of mutational signatures in human cancer. Nature. 2020;578(7793):94-101. doi:10.1038/s41586-020-1943-3
- Dousset L, Boussemart L, Robert C, et al. Tumour mutational burden and response to PD-1 inhibitors: an analysis of 89 cases of metastatic melanoma. Ann Oncol. 2019;30(suppl 5):V553. doi:10.1093/annonc/mdz255.043
- Wu Y, Xu J, Du C, et al. The predictive value of tumor mutation burden on efficacy of immune checkpoint inhibitors in cancers: a systematic review and meta-analysis. Front Oncol. 2019;9:1161. doi:10.3389/fonc.2019.01161
- Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189-2199. doi:10.1056/NEJMoa1406498
- Johnson DB, Frampton GM, Rioth MJ, et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol Res. 2016;4(11):959-967. doi:10.1158/2326-6066.CIR-16-0143
- Krieger T, Pearson I, Bell J, et al. Targeted literature review on use of tumor mutational burden status and programmed cell death ligand 1 expression to predict outcomes of checkpoint inhibitor treatment. Diagn Pathol. 2020;15(1):6. doi:10.1186/s13000-020-0927-9
- Yaghmour G, Pandey M, Ireland C, et al. Role of genomic instability in immunotherapy with checkpoint inhibitors. Anticancer Res. 2016;36(8):4033-4038.
- Roszik J, Haydu LE, Hess KR, et al. Novel algorithmic approach predicts tumor mutation load and correlates with immunotherapy clinical outcomes using a defined gene mutation set. BMC Med. 2016;14(1):168. doi:10.1186/s12916-016-0705-4
- Forschner A, Battke F, Hadaschik D, et al. Tumor mutation burden and circulating tumor DNA in combined CTLA-4 and PD-1 antibody therapy in metastatic melanoma – results of a prospective biomarker study. J Immunother Cancer. 2019;7(1):180. doi:10.1186/s40425-019-0659-0
- Miao D, Margolis CA, Vokes NI, et al. Genomic correlates of response to immune checkpoint blockade in microsatellite-stable solid tumors. Nat Genet. 2018;50(9):1271-1281. doi:10.1038/s41588-018-0200-2
- Garon EB, Rizvi NA, Hui R, et al; KEYNOTE-001 Investigators. Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med. 2015;372(21):2018-2028. doi:10.1056/NEJMoa1501824
- Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124-128. doi:10.1126/science.aaa1348
- Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti–programmed cell death (PD)-1 and anti–programmed death-ligand 1 (PD-L1) blockade in patients with non–small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633-641. doi:10.1200/JCO.2017.75.3384
- Carbone DP, Reck M, Paz-Ares L, et al; CheckMate 026 Investigators. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. doi:10.1056/NEJMoa1613493
- Chang H, Sasson A, Srinivasan S, et al. Bioinformatic methods and bridging of assay results for reliable tumor mutational burden assessment in non-small-cell lung cancer. Mol Diagn Ther. 2019;23(4):507-520. doi:10.1007/s40291-019-00408-y
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031. doi:10.1056/NEJMoa1910231
- Califano R, Lal R, Lewanski C, et al. Patient selection for anti-PD-1/PD-L1 therapy in advanced non-small-cell lung cancer: implications for clinical practice. Future Oncol. 2018;14(23):2415-2431. doi:10.2217/fon-2018-0330
- Willis C, Fiander M, Tran D, et al. Tumor mutational burden in lung cancer: a systematic literature review. Oncotarget. 2019;10(61):6604-6622. doi:10.18632/oncotarget.27287
- Zhang C, Li Z, Qi F, et al. Exploration of the relationships between tumor mutation burden with immune infiltrates in clear cell renal cell carcinoma. Ann Transl Med. 2019;7(22):648. doi:10.21037/atm.2019.10.84
- Motzer RJ, Rini BI, McDermott DF, et al; CheckMate 214 Investigators. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370-1385. doi:10.1016/S1470-2045(19)30413-9. Published corrections appear in Lancet Oncol. 2019;20(10):e559; and Lancet Oncol. 2020;21(6):e304.
- Rini BI, Plimack ER, Stus V, et al; KEYNOTE-426 Investigators. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116-1127. doi:10.1056/NEJMoa1816714
- Ma Y, Feng XF, Yang WX, You CG. Exploring the pathological mechanism of bladder cancer based on tumor mutational burden analysis. Biomed Res Intl. 2019;2019:1093815. doi:10.1155/2019/1093815
- Lv J, Zhu Y, Ji A, et al. Mining TCGA database for tumor mutation burden and their clinical significance in bladder cancer. Biosci Rep. 2020;40(4):BSR20194337. doi:10.1042/BSR20194337
- Thomas A, Routh ED, Pullikuth A, et al. Tumor mutational burden is a determinant of immune-mediated survival in breast cancer. Oncoimmunology. 2018;7(10):e1490854. doi:10.1080/2162402X.2018.1490854
- Pai SG, Carneiro BA, Chae YK, et al. Correlation of tumor mutational burden and treatment outcomes in patients with colorectal cancer. J Gastrointest Oncol. 2017;8(5):858-866. doi:10.21037/jgo.2017.06.20
- Cao D, Xu H, Xu X, et al. High tumor mutation burden predicts better efficacy of immunotherapy: a pooled analysis of 103078 cancer patients. Oncoimmunology. 2019;8(9):e1629258. doi:10.1080/2162402X.2019.1629258
- Peltomäki P. Update on Lynch syndrome genomics. Fam Cancer. 2016;15(3):385-393. doi:10.1007/s10689-016-9882-8
- Ligtenberg MJL, Kuiper RP, Chan TL, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat Genet. 2009;41(1):112-117. doi:10.1038/ng.283
- Veigl ML, Kasturi L, Olechnowicz J, et al. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Nation Acad Sci U S A. 1998;95(15):8698-8702. doi:10.1073/pnas.95.15.8698
- Palles C, Cazier J-B, Howarth KM, et al; CORGI Consortium; WGS500 Consortium. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45(2):136-144. doi:10.1038/ng.2503
- Shlien A, Campbell BB, De Borja R, et al; Biallelic Mismatch Repair Deficiency Consortium. Combined hereditary and somatic mutations of replication error repair genes result in rapid onset of ultra-hypermutated cancers. Nat Genet. 2015;47(3):257-262. doi:10.1038/ng.3202
- Lynch HT, Lynch PM, Lanspa SJ, et al. Review of the Lynch syndrome: history, molecular genetics, screening, differential diagnosis, and medicolegal ramifications. Clin Genet. 2009;76(1):1-18. doi:10.1111/j.1399-0004.2009.01230
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi:10.1056/NEJMoa1500596
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi:10.1126/science.aan6733
- Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1-10. doi:10.1200/JCO.19.02105
- Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008-1019. doi:10.1001/jamaoncol.2019.0393
- Skoulidis F, Arbour KC, Hellmann MD, et al. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. J Clin Oncol. 2019;37(suppl 15; abstr 102). doi:10.1200/JCO.2019.37.15_suppl.102
- Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 2016;76(5):999-1008. doi:10.1158/0008-5472.CAN-15-1439
- Kadara H, Choi M, Zhang J, et al. Whole-exome sequencing and immune profiling of early-stage lung adenocarcinoma with fully annotated clinical follow-up Ann Oncol. 2017;28(1):75-82. doi:10.1093/annonc/mdw436. Published correction appears in Ann Oncol. 2018;29(4):1072.
- Skoulidis F, Goldberg ME, Greenawalt DM, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 2018;8(7):822-835. doi:10.1158/2159-8290.CD-18-0099
- George S, Miao D, Demetri GD, et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity. 2017;46(2):197-204. doi:10.1016/j.immuni.2017.02.001
- Peng W, Chen JQ, Liu C, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016;6(2):202-216. doi:10.1158/2159-8290.CD-15-0283
- Restifo NP, Marincola FM, Kawakami Y, et al. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88(2):100-108. doi:10.1093/jnci/88.2.100
- Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819-829. doi:10.1056/nejmoa1604958
- Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Ann Rev Immunol. 1997;15:563-591. doi:10.1146/annurev.immunol.15.1.563
- Sanghoon Shin D, Zaretsky JM, Escuin-Ordinas H, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Disc. 2017;7(2):188-201. doi:10.1158/2159-8290.CD-16-1223
- Coulie PG, Van den Eynde BJ, van der Bruggen P, Boon T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer. 2014;14(2):135-146. doi:10.1038/nrc3670
- Cohen CJ, Gartner JJ, Horovitz-Fried M, et al. Isolation of neoantigen-specific T cells from tumor and peripheral lymphocytes. J Clin Invest. 2015;125(10):3981-3991. doi:10.1172/JCI82416
- Xin Yu J, Hubbard-Lucey VM, Tang J. The global pipeline of cell therapies for cancer. Nat Rev Drug Discov. 2019;18(11):821-822. doi:10.1038/d41573-019-00090-z
- George AP, Kuzel TM, Zhang Y, Zhang B. The discovery of biomarkers in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:484-497. doi:10.1016/j.csbj.2019.03.015
- Krieg C, Nowicka M, Guglietta S, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;24(2):144-153. doi:10.1038/nm.4466
- Subrahmanyam PB, Dong Z, Gusenleitner D, et al. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J Immunother Cancer. 2018;6(1):18. doi:10.1186/s40425-018-0328-8
- Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359(6371):91-97. doi:10.1126/science.aan3706
- Zaragoza J, Caille A, Beneton N, et al. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2016;174(1):146-151. doi:10.1111/bjd.14155
- Halvorsen AR, Sandhu V, Sprauten M, et al. Circulating microRNAs associated with prolonged overall survival in lung cancer patients treated with nivolumab. Acta Oncologica. 2018;57(9):1225-1231. doi:10.1080/0284186X.2018.1465585
- Qiu P, Poehlein CH, Marton MJ, et al. Measuring tumor mutational burden (TMB) in plasma from mCRPC patients using two commercial NGS assays. Sci Rep. 2019;9(1):114. doi:10.1038/s41598-018-37128-y
- Solomon BL, Garrido-Laguna I. TIGIT: a novel immunotherapy target moving from bench to bedside. Cancer Immunol Immunother. 2018;67(11):1659-1667. doi:10.1007/s00262-018-2246-5
- Sullivan RJ, Lim EA, Sharma M, Shepard DR, et al. A phase I study evaluating COM701 monotherapy and in combination with nivolumab in patients with advanced solid malignancies. J Clin Oncol. 2020;38(suppl 5; abstr TPS23). doi:10.1200/JCO.2020.38.5_suppl.TPS23
